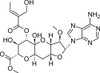
Herbicidin A is an antibiotic with selective herbidical activity against dicots. It was originally isolated from Streptomyces saganonensis in a soil sample by Sankyo (Japan) in 1976. It is the major analog of a family of adenosine-based nucleosides containing a complex tricyclic saccharide. Herbicidin A is soluble in ethanol, methanol, DMF and DMSO.
| Mechanism of Action | The mode of action and broader pharmacology of Herbicidin A has received little attention due to its restricted availability. |
| Microbiology Applications | Fermentation parameters for Herbicidin A production in submerged culture of Streptomyces scopuliridis M40 were investigated. Bioproduction was successfully scaled up from a 5-L jar to a 500-L pilot vessel (Ha et al, 2017). |
| Plant Biology Applications | Bioherbicides include phytopathogenic microorganisms or microbial compounds useful for weed control. Bioherbicides have several advantages: 1) high specificity, 2) absence of environmental residue; and 3) reduction of resistant weed populations (Hoagland RE et al, 2007).
Herbicidin A is both a herbicide against weeds affecting dicot plants and an antibiotic against phytopathogens. |
| References |
Arai M, Haneishi T, Kitahara N, Enokita R and Kawakubo K (1976) Herbicidins A and B, two new antibiotics with herbicidal activity. I. Producing organism and biological activities.. J. Antibiot. (Tokyo) 29(9): 863-869 Ha S et al (2017) Optimization of Herbicidin A production in submerged culture of Streptomyces scopuliridis M40. J. Micrrobiol. Biotechnol. 27(5):947-955 Haneishi T. et al. (1976) Herbicidin A and B, two new antibiotics with herbicidal activity. II. Fermentation, isolation and physico-chemical characterization. Antibiot. (Tokyo) 29:870 Hoagland RE, Boyette CD and Weaver MA (2007) Bioherbicides: Research and risks. Toxin Rev. 26:313-342 Lin G, Romo AJ, Liem PH, Chen Z and Liu H (2017) Identification and interrogation of the herbicidin biosynthetic gene cluster: First insight into the biosynthesis of arare undecose nucleoside antibiotic. J. Am. Chem. Soc. 139(46):16450-16453 PMID 29111702 |