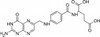
Folic Acid, USP (vitamin B9) and the resulting metabolites are essential to a number of organisms. Folic Acid is slightly soluble in water and soluble in dilute acidic solutions and alkaline solutions.
| Mechanism of Action | Cellular enzymes convert folic acid into dihydrofolic acid which is used as a precursor for a number of compounds including tetrahydrofolate (THF) which are involved in DNA repair and synthesis because of its role in purine synthesis. |
| Microbiology Applications | Folic Acid is frequently used in cell culture to provide tetrahydrofolates and other essential metabolites. |
| Eukaryotic Cell Culture Applications | Folic Acid has been used to research the influence of vitamins and amino acids on the growth of hybridomas and their influence on monoclonal antibody production. |
| Molecular Formula | C19H19N7O6 |
| Solubility | Acid solutions (dilute): Soluble Alkaline solutions: Soluble Methanol: Slightly soluble Water: Slightly soluble |
| References |
Aaronson S and Rodriguez E (1958) Relationship between purines Folic Acid-vitamins in the micrococcus, Gaffkya homari. 75(6):660-665 Ducommun P, Ruffieux PA, von Stockar U and Marison I (2001) The role of vitamins and amino acids on hybridoma growth and monoclonal antibody production. Cytotechnol. 37(2), 65–73 PMID 19002903 |