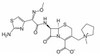
Cefepime Hydrochloride, USP is the hydrochloride salt of Cefepime. The addition of the hydrochloride salt enhances aqueous solubility. It is similar in potency to Cefepime, a broad-spectrum, fourth-generation cephalosporin antibiotic that was patented in 1982 by Bristol-Myers Squibb. Cefepime is bactericidal against a wide range of Gram-positive and Gram-negative bacteria, disrupting bacterial cell wall synthesis. It appears to be intrinsically more resistant to hydrolysis by β-lactamases and is commonly used in antimicrobial susceptibility testing. Cefepime Hydrochloride is soluble in water.
We also offer:
- Cefepime (C008)