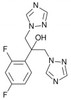
Fluconazole, USP is broad-spectrum fungistatic triazole antifungal. Fluconazole targets ergosterol synthesis in a wide range of fungal species such as Candida spp., Cryptococcus neoformans and dermatophytes.
Fluconazole is practically insoluble in water.
| Mechanism of Action | Fluconazole interferes with the conversion of lanosterol to ergosterol, an essential cell membrane component. The inhibition of ergosterol synthesis increases cell permeability which disrupts normal cellular function.
Resistance to antifungals can include alteration in drug target, alteration in sterol biosynthesis, reduction in the intercellular concentration of target enzyme, or overexpression of the antifungal drug target (Ghannoum and Rice, 1999). It is a fungal cytochrome P-450 inhibitor, specifically sterile C-14-α-demethyllation. Resistance in C. albicans is due to mutations in the ERG11 gene which codes for C-14-α-demethyllation. Mutations in this gene prevent the compound from binding while still allowing binding to the enzyme’s natural substrate lanosterol. It also inhibits the human cytochrome P450 system, particularly the isozyme CYP2C9. Therefore, in theory, compounds that are metabolized by this enzyme tend to increase in concentration. |
| Spectrum | Broad-spectrum including Candida spp. (excluding C. krusei and C. glabrata) and Cryptococcus neoformans. Also effective against dermatophytes such as Microsporum, Epidermophyton and Trichophyton. |
| Impurity Profile | Impurity A: ≤0.2% Impurity B: ≤0.1% Impurity C: ≤0.2% Total unknown impurities: ≤0.3% Total impurities: ≤1.5% |
| Microbiology Applications | Fluconazole is commonly used in clinical in vitro microbiological antimicrobial susceptibility tests (panels, discs, and MIC strips) against fungal isolates. Medical microbiologists use AST results to recommend antibiotic treatment options. Representative MIC values include:
|
| Eukaryotic Cell Culture Applications | There is no relationship between in vitro and in vivo activities for this compound, thus antifungal efficacy was mainly proved in animal models, and has low toxicity (Vincent-Ballereau et al, 1991). |
| Molecular Formula | C13H12F2N6O |
| Solubility | Practically insoluble in water (0.001 mg/ml). Sparingly soluble in ethanol (20 mg/ml) and DMSO (33 mg/ml). |
| Impurity Profile | Impurity A: Not more than 0.2% Impurity B: Not more than 0.1% Impurity C: Not more than 0.2% Total unknown impurities: Not more than 0.3% Total impurities: Not more than 1.5% |
| References |
Ghannoum MA and Rice LB (1999) Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12 (4):501-517 PMID 10515900 Re JH et al (1997) Development of interpretive breakpoints for antifungal susceptibility testing: Conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida Infections. Clin. Infect. Dis. 24(2):235–247 Sobel, JD and Akins RA (2004) Fungicidal activity of fluconazole against Candida albicans in a synthetic vagina-simulative medium. Antimicrob. Agents Chemother. 48(1):161-167 Vincent-Ballereau FN, Patey ON, Lafaix C (1991) Fluconazole. Review and situation among antifungal drugs in the treatment of opportunistic mycoses of human immuno-deficiency virus infections. Pharm Weekbl Sci. 13(2):45-57 PMID 1870943 |