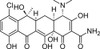
Chlortetracycline was the first reported member of the tetracycline class, isolated from Streptomyces aureofaciens in 1948. Chlortetracyclines heralded the early wave of antibiotic discoveries from microbes and after 50 years are still widely used as pharmaceuticals. Chlortetracycline is a pigment and, like most pigments, is extremely sensitive to environmental and storage conditions. Commercial chlortetracycline may contain significant levels of degradation products.
Chlortetracycline is soluble in ethanol, methanol, DMF and DMSO.
Chlortetracycline is soluble in ethanol, methanol, DMF and DMSO.
| Mechanism of Action | The mechanism of chlorotetracycline involves entering a cell and binding to the 30s ribosomal subunit preventing peptide elongation and ultimately inhibiting protein synthesis. Resistance to chlorotetracycline can be a result of inactivation by cell enzymes or pumping the antibiotic out of the cell upon entering. |
| Molecular Formula | C22H23ClN2O8 |
| References | Aureomycin, a new antibiotic. Broschard R.W. et al. Science 1949, 109, 199. Chemical stability of chlortetracycline and chlortetracycline degradation products and epimers in soil interstitial water. Soeborg T. et al. Chemosphere 2004, 57, 1515. |