Kanamycin Acid Sulfate, BP is an aminoglycoside antibiotic often used to select for bacteria which have been successfully transformed with a plasmid conferring kanamycin resistance. Kanamycin is very soluble in aqueous solution at 92.3 mg/mL.
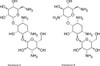
Standard grade Kanamycin is composed of a mixture of three different fractions: Kanamycin A, B, and C. Kanamycin A is the major component of the complex, and Kanamycin B (II) and C (III) are minor components.
We also offer:
- Kanamycin Sulfate, USP (K008)
- Kanamycin Acid Sulfate, for BioProcessing (K012)
- Kanamycin A sulfate, EvoPure® (K013)
- Kanamycin B sulfate, EvoPure® (K014)
EvoPure® products are purified single antibiotic fractions, most >99% pure. High purity EvoPure® kanamycin products can be used to analyze the specific effects of individual kanamycin fractions.
| Mechanism of Action | Aminoglycosides target the 30S ribosomal subunit resulting in an inability to read mRNA ultimately producing a faulty or nonexistent protein. |
| Spectrum | Kanamycin is a broad spectrum antibiotic; however, it is mostly used against aerobic Gram-negative bacteria. |
| Microbiology Applications | Kanamycin Acid Sulfate is commonly used as a selective agent to select for resistant mammalian, fungal, or bacterial cells that contain the kanMX marker or other Kanamycin resistance genes. Kanamycin Acid Sulfate is typically used at a concentration of 50 µg/mL.
Pryjma et al.used Kanamycin Sulfate (TOKU-E) to select for transformed kanamycin resistant Campylobacter jejuni cells in: "FdhTU-modulated formate dehydrogenase expression and electron donor availability enhance recovery of Campylobacter jejuni following host cell infection"
Media SupplementsKanamycin can be used as a selective agent in several types of isolation media: Kanamycin Aesculin Azide Agar - Enterococci isolation in food Perfringens Agar - SFP and TSC selective supplements for the isolation of Clostridium perfringens |
| Plant Biology Applications | Kanamycin is often used in the Agrobacterium mediated transformation while using the npt II gene as selection marker. Kaur and Bansal (2010) used kanamycin in combination with cefotaxime to control bacterial growth while transforming tomatoes. |
| Molecular Formula | C18H36N4O11 · 2 H2SO4 |
| References |
Davis BD (1987) Mechanism of Bactericidal Action of Aminoglycosides. Microbiol. Rev. 51(3):341-350 Claes PJ, Dubost M and Vanderhaeghe H (1977) Kanamycin sulfate. Analytic. Profiles Drug Sub. 6:259-296 |
| MIC | Bacillus subtilis| 3.9| Candida albicans| 2000| Escherichia coli| 31.2| Salmonella Enteritidis| 62.5| Staphylococcus aureus| 31.2| |