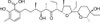
Lasalocid Sodium is the salt form of Lasalocid, a carboxylic polyether ionophore. The compound has anticancer properties and antibacterial activity. Lasalocid is isolated from Streptomyces lasaliensis, first reported in 1951. It is also known as a coccidiostat; developed for the treatment of coccidia in animals, it stops coccidia from reproducing.
Lasalocid is a mixture of several closely related homologues: A, B, C, D and E. Lasalocid Sodium is primarily made up of Lasalocid A, with the other analogs (B, C, D, E) making up no more than 10% of the total weight of the active substance.
Lasalocid Sodium is soluble in ethanol, methanol, DMF and DMSO. It has limited water solubility.
| Mechanism of Action |
As an ionophore, the compound can increase the permeability of biological (or artificial) lipid membranes to specific ions. Lasalocid Sodium is an ionophore that can form complexes with cations and transport through lipid membranes. It can also transport larger organic cations. In cancer researchstudies, researchers found lasalocid induces autophagy through microtubule-associated protein 1 light chain 3 (LC-3)-II conversion. The autophagic properties were mediated by production of reactive oxygen species (ROS), confirmed by using a ROS inhibitor. |
| Spectrum | Lasalocid Sodium is effective against Gram-positive bacteria and protozoa. Examples of Gram-positive bacteria that are sensitive to the compound are Fusobacterium, Bifidobacterium, Eubacterium, Clostridium, and Lactobacillus. |
| Molecular Formula | C34H53NaO8 |
| References |
Berger J et al (1951) The Isolation of three new crystalline antibiotics from Streptomyces. J. Am. Chem. Soc. 73:5295 Hucyński A et al (2013) X-ray crystallographic, FT-IR and NMR studies as well as anticancer and antibacterial activity of the salt formed between ionophore antibiotic Lasalocid Acid and amines. J. Molec. Struc. 1032:69-77 Kim K et al (2017) Lasalocid induces cytotoxic apoptosis and cytoprotective autophagy through reactive oxygen species in human prostate cancer PC-3 cells. Biomed. & Pharmacol. 88:1016-1024 Kinsel JF (1982) The effect of amine structure on complexation with Lasalocid in model membrane systems. I. Identification of charged complexes in lipid bilayer membranes. Biochim Biophys Acta. 684:233 PMID 7055564 Shen C and Patel DJ (1977) Biogenic amine-ionophore interactions: Structure and dynamics of Lasalocid (X537A) complexes with phenethylamines and catecholamines in nonpolar solution. Proc Natl Acad Sci USA. 74:4734 Stromberg BE, Schlotthauer JC, Armstrong BD, Brandt WE and Liss C (1982) Efficacy of Lasalocid Sodium against coccidiosis (Eimeria zuernii and Eimeria bovis) in calves. Am. J. Vet Res. 43(4):583-585 PMID 7073077 Westley JW (1970) Structure of antibiotic X-537A. Chem. Soc. D, 71 Westley JW et al (1970) Biosynthesis of antibiotic X-537A. Chem. Soc. D, 1467 |