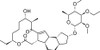
3'-Ethoxy-5,6-dihydrospinosyn J pseudoaglycone is an acid degradation product produced by selective hydrolysis of the more labile forosamine saccharide in the 17-position of 3'-ethoxy-5,6-dihydrospinosyn J, the major component of the commercial product, Spinetoram. The compound is only weakly active as an insecticide as the forosamine moiety is considered essential for potent activity. Despite the importance of spinosyns as agro-chemical insecticides, there are few published reports of the biological activity of this natural product in the environment.
The product is soluble in ethanol, methanol, DMF and DMSO.
| References | Creemer LC et al (1998) Conversion of Spinosyn A and Spinosyn D to their respective 9- and 17-pseudoaglycones and their aglycones. J Antibiot. 51:795 Kirst HA (2010) The Spinosyn family of insecticides: Realizing the potential of natural products research. J. Antibiot. 63:101 |