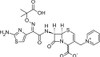
Ceftazidime, Delta-3-Isomer, EvoPure is an isomer and degradation product of Ceftazidime. It can be used as a reference standard to trace Ceftazidime. This isomer was formerly known as Delta-2.
Ceftazidime is a broad-spectrum, third-generation, β-lactam cephalosporin that interferes with bacterial cell wall synthesis. Patented in 1979 by Glaxo Group, Ceftazidime came into commercial use in 1984. It is sparingly soluble in aqueous solution.
EvoPure products are highly purified compounds that can be used for in vitro applications like analytical reference standards, upstream pharmaceutical product manufacturing, gene selection, and toxicity studies.
We also offer:
| Mechanism of Action | Like β-lactams, cephalosporins interfere with PBP (penicillin binding protein) activity involved in the final phase of peptidoglycan synthesis. PBP’s are enzymes which catalyze a pentaglycine crosslink between alanine and lysine residues providing additional strength to the cell wall. Without a pentaglycine crosslink, the integrity of the cell wall is severely compromised and ultimately leads to cell lysis and death. Resistance to cephalosporins is commonly due to cells containing plasmid-encoded β-lactamases, however, Ceftazidime is stable in the presence of β-lactamases. |
| Spectrum | Ceftazidime is broad-spectrum, targeting both Gram-negative and Gram-positive bacteria, but is most effective for Gram-negative strains including Pseudomonas aeruginosa and Enterobacteriaceae (including β-lactamase positive strains). It is also used for against Streptococcus pneumoniae, and S. pyogenes. |
| Microbiology Applications | Ceftazidime is commonly used in clinical in vitro microbiological antimicrobial susceptibility tests (panels, discs, and MIC strips) against Gram-negative microbial isolates. Medical microbiologists use AST results to recommend antibiotic treatment options. Representative MIC values include:
|
| Molecular Formula | C22H22N6O7S2 · 5H2O |
| References | Georgopapadakou NH (1992) Mechanisms of action of cephalosporin 3'-quinolone esters, carbamates, and tertiary amines in Escherichia coli. Antimicrob. Agents. Chemother. 37(3): 559-565 PMID 8384817 Fischer J and Ganellin R (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 495 Hobi R et al (2001) Anti-HIV-1 activity in vitro of Ceftazidime degradation products. Antivir. Chem. and Chemother. 12(2):109-118 Hsu Y et al (2014) Biodegradable drug-eluting nanofiber-enveloped implants for sustained release of high bactericidal concentrations of vancomycin and ceftazidime: In vitro and in vivo studies. Int. J. Nanomed. 9:4347-4355 |