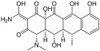
Doxycycline Hydrate is a third-generation tetracycline and matrix metalloproteinase inhibitor, synthesized in 1958. It has broad-spectrum antibacterial and antiprotozoan activity, and interferes with bacterial protein synthesis. It is soluble in ethanol, methanol, DMF and DMSO.
We also offer:
| Mechanism of Action | Doxycycline Hydrate is prepared by hydrogenolysis of oxytetracycline to remove the 6-hydroxy group. Tetracycline antimicrobials bind to the bacterial 30S ribosomal subunit interfering with tRNA/mRNA interaction, ultimately inhibiting protein synthesis. Tetracyclines can inhibit the MMP enzyme family and inhibit mitochondrial biogenesis. |
| Spectrum | Doxycycline Hydrate has broad-spectrum activity against Gram-positive and Gram-negative bacteria, and antiprotozoan activity. |
| Microbiology Applications | Doxycycline is commonly used in clinical in vitro microbiological antimicrobial susceptibility tests (panels, discs, and MIC strips) against Gram-positive, Gram-negative, and certain Mycoplasma species. Medical microbiologists use AST results to recommend antibiotic treatment options. Representative MIC values include:
|
| Eukaryotic Cell Culture Applications | Doxycycline is routinely used for gene selection. For additional information on your cell culture needs, please visit our Cell Culture Database. |
| Cancer Applications | Doxycycline was able to inhibit cancer stem cell progression across an entire panel of 12 different tumor cell lines representing different cancer types (DCIS, breast, lung, ovarian, pancreatic, prostate, glioblastoma, melanoma (Lamb et al, 2015). Doxycycline can eradicate cancer stem cells in breast cancer patients in vivo. Authors found a quantitative decease in CD44 and ALDH1 expression, biomarkers of ‘stemness’. This is promising work in using cancer stem cells for cancer prevention, and is an excellent candidate for compound repurposing (Scatena C et al, 2018). |
| Molecular Formula | C22H24N2O8 · H2O |
| References | Franco et al (2006) Doxycycline alters vascular smooth muscle cell adhesion, migration, and reorganization of fibrillar collagen matrices. Am. J. Pathol 16895):1697-1709 PMID 16651635 Gossen M et al (1995) Transcriptional activation by tetracyclines in mammalian cells. Science 268(5218):1766-1769 PMID 7792603 Lamb R et al (2015) Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: Treating cancer like an infectious disease. Oncotarget. 6(7):4569-84 PMID 25625193 Rosenblatt JE et al (1966) Comparison of in vitro activity and clinical pharmacology of Doxycycline with other tetracyclines. Antimicrob. Agents Chemother. 6:134 PMID 4964450 Scatena C et al (2018) Doxycycline, an inhibitor of mitochondrial biogenesis, effectively reduces cancer stem cells (CSCs) in early breast cancer patients: A clinical pilot study. Front. Oncol. 8:452 PMID 30364293 Product ReferenceDoxycycline (TOKU-E) was used in methacrylate-based copolymer films and studied their effects on biofilm formation: "Prevention of Biofilm Formation by Methacrylate-Based Copolymer Films Loaded With Rifampin, Clarithromycin, Doxycycline Alone or in Combination." (Rose et al) |