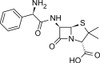
Ampicillin Trihydrate, USP is a member of the β-lactam family similar in structure to penicillin. Ampicillin Trihydrate is slightly soluble in water (10 mg/mL) and soluble in 1 N HCl (50 mg/mL).
We also offer:
- Ampicillin/Sulbactam (2:1) (A071)
- Ampicillin Anhydrous (A043)
- Ampicillin Sodium (A042)
- Ampicillin Trihydrate, EP (A020)
Ampicillin Trihydrate, USP conforms to United States Pharmacopoeia specifications.
| Mechanism of Action | Like all β-lactams, Ampicillin interferes with PBP (penicillin binding protein) activity otherwise involved in the final phase of peptidoglycan synthesis. PBP’s are enzymes which catalyze a pentaglycine crosslink between alanine and lysine residues. Without a pentaglycine crosslink, the integrity of the cell wall is severely compromised ultimately leading to cell lysis. |
| Spectrum | Ampicillin, USP targets Gram-negative non-ESBL (Extended Spectrum β-lactamase) bacteria including Staphylococcus and Streptococcus species and medically important enteric pathogens such as Shigella and Salmonella. Interestingly, ampicillin has been found to be effective against certain β-lactam sensitive VRE or vancomycin resistant Enterococcus; a glycopeptide antibiotic resistant "superbug." Resistance to Ampicillin is routinely utilized as a selectable marker to confirm successful cell transformation. |
| Microbiology Applications | Ampicillin Trihydrate is often used to select for cells that have been transformed with a plasmid containing the ampR gene which confers resistance to Ampicillin.
Media SupplementsAmpicillin Trihydrate can be used as a selective agent in several types of isolation media: Aeromonas Medium Base - Ampicillin Selective Supplement |
| Eukaryotic Cell Culture Applications | Ampicillin is routinely used to select for cells containing the pcDNA3.1 and pEAK10 resistance plasmids in cell line A904L at an effective concentration of 50 µg/mL. For additional information, please visit our cell-culture database. |
| Molecular Formula | C16H19N3O4S·3H2O |
| Solubility | Slightly soluble in water (10 mg/mL) and soluble in 1 N HCl (50 mg/mL) |
| References |
Pitout JD, Sanders CC, Sanders WE (1997) Antimicrobial resistance with focus on beta-lactam resistance in gram-negative bacilli. Am. J. Med 103(1):51-59 PMID 9236486 Waxman DJ and Strominger JL (1983) Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Ann. Rev. Biochem 52:825-869 PMID 6351730 Yang W, Zhang L, Lu Z, Tao W, Zhai Z (2001) A new method for protein coexpression in Escherichia coli using two incompatible plasmids. Protein. Expr. Purif. 22(3):472-478 PMID 11483011 |