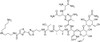
Bleomycin A5 Hydrochloride is part of the Bleomycin complex. It is an anti-neoplastic glycoprotein antibiotic used in cancer research. The Bleomycins were originally isolated for their broad spectrum antibacterial activity.
We also offer:
- Bleomycin Sulfate (B005)
- Bleomycin A2 Sulfate, Evopure® (B019)
- Bleomycin B2 Sulfate, Evopure® (B020)
- Bleomycin (B053)
| Mechanism of Action | Bleomycin's anticancer activities include the increase of caspase-3 and p53, and the inhibition of telomerase activity leading to apoptosis. The antitumor activity derives from their ability to effect DNA cleavage in cancer cells. |
| Microbiology Applications | Bleomycin A5 Hydrochloride is used in gene selection using the Sh ble gene which confers resistance to Bleomycin and Bleomycin A5. |
| Cancer Applications | Bleomycin contains a disaccharide moiety composed of 2 unusual sugars, L-gulose and 3-O-carbamoyl-D-mannose. Bleomycin could be regarded as a modular system composed of a tumor-targeting agent (the dissacharide moiety) and a tumoricidal agent (deglycobleomycin). The disaccharide moiety is responsible for the tumor cell targeting properties of Bleomycin. Bleomycin analogs were prepared, the glycosylated analogs were more cytotoxic to cultured DU145 prostate cancer cells. These findings establish a role for the Bleomycin disaccharide in tumor targeting/uptake and suggest that the disaccharide moiety may be capable of delivering other cytotoxins to cancer cells (Schroeder et al, 2014). Bleomycin is used in combination with other antineoplastic agents in studying lymphomas, testicular carcinomas, and squamous cell carcinomas. In this report, we found that the human L-carnitine transporter (hCT2) is involved in Bleomycin-A5 uptake. NT2/D1 human testicular cancer cells which highly express hCT2 are very sensitive to Bleomycin-A5. Data suggest that hCT2 can mediate the uptake of Bleomycin A5 (Aouida M et al, 2010). In cell culture experiments with Bleomycins and BLM carbohydrates conjugated to microbubbles it has been demonstrated that Bleomycins are tumor-seeking molecules. Biotinylate Bleomycin A5 was attached to microbubbles, and a conjugate-containing solution was passed over a monolayer of MCF-7 cells. The microbubbles adhered to the MCF-7 cells. The conjugate did not bind to a normal breast cell line or to matched noncancer cell lines. No binding occurred if the microbubbles lacked conjugated Bleomycin A5 or if the microbubble lacked the carbohydrate moiety (Chapuis et al, 2009). A well-known characteristic of tumor cells is the Warburg effect, that is the propensity of tumor cells to produce increased ATP via glycolysis rather than by mitochondrial oxidative phosphorylation. The shift to glycolysis is accompanied by upregulation of glucose transporters to provide the greater amounts of glucose needed to support increased glycolysis. If authors treated two normal cell lines (normal lunch WI-38 cells and normal kidney CCD-1105 KIDTr cells) with the inhibitor rotenone, (a mitochondrial complex 1 inhibitor), this forced these cells to use increase glycolysis in the same fashion as tumor cells and this resulted in an enhanced ability to incorporate BLM-Cy5. The finding implies that the Bleomycin saccharide moiety may be able to deliver other cytotoxins selectively to tumor cells (Mobasheril, 2005). In a study of the Bleomycin A5 was applied on a human oral epidermoid carcinoma cell line (KB) to study the signal transduction pathways that might exert apoptotic effects of Bleomycin A5 on tumor cells. Researchers found that c-Jun N-terminal kinases (JNK) were activated, suggesting that JNK plays a role in apoptosis. In addition, combining Bleomyin A5 with MAP kinase-ERK kinase inhibitor could lead to enhanced apoptosis (Yang L et al, 2004). |
| Molecular Formula | C57H89N19O21S2 • HCl |
| References | Aouida M, Poulin P and Ramotar (2010) The human carnitine transporter SLC22A16 mediates high affinity uptake of the anticancer polyamine analogue Bleomycin-A5. J. Biol. Chem. 285:6275-6284. Burger RM, Paisach J, and Horwitz SB (1981) Mechanism of Bleomycin action: In vitro studies. Life Sci. 28(7):715-727 PMID 6164898Chapuis J, Schmaltz RM, Tsosie KS, Belohlavek M and Hecht SM (2009) Carbohydrate dependent targeting of cancer cells by bleomycin-microbubble conjugates. J. Am. Chem. Soc. 131(7):2438-2439 Dorr RT (1991) Bleomycin Pharmacology: Mechanism of action and resistance, and clinical pharmacokinetics. Semin. Oncol. 19(2): 3-8. PMID 1384141Fujiwara Y and Kondo T (1973) Strand-scission of HeLa cell deoxyribonucleic acid by Bleomycin in vitro and in vivo. Biochem. Pharmacol 22(3):323-333 PMID 4119868 Huang, YD et al (2015). Effects of Bleomycin A5 on caspase-3, P53, bcl-2 expression and telomerase activity in vascular endothelial cells. Ind. J. Pharmacol 47(1):55 Kross et al. (1982) Structural basis for the deoxyribonucleic acid affinity of Bleomycins. Biochem. 21: 3711-3721 PMID 6180763 Luo, Q and Zhao F (2011) The effects of Bleomycin A5 on infantile maxillofacial haemangioma. Head Face Med. 7(1), doi:10.1186/1746-160x-7-11 PMID 21736714
Schroeder BR et al (2014) The disaccharide moiety of bleomycin facilitates uptake by cancer cells. J. Am. Chem Soc. 136(39):13641-13656 Takita T et al (1972) Chemistry of Bleomycin. IX. The structures of Bleomycin and phleomycin. J. Antibiot. (Tokyo) 25:755-757 PMID 4119701 Yang L, Yang S, Tai K, Chou M and Yang J (2004) MEK inhibition enhances Bleomycin A5-induced apoptosis in an oral cancer cell line: Signaling mechanisms and therapeutic opportunities. J. Oral Path. and Med. 33(1):37-45
|