We often get asked what is the difference between purity and potency for antibiotic compounds.
Purity is a measure of how chemically pure the antibiotic is. This measurement is needed to ensure it is not contaminated during the manufacturing process. It can also be used to confirm its identification. Purity means freedom from extraneous matter like residual impurities, volatiles, and pyrogenic substances. Purity is usually measured by analytical methods such as high performance liquid chromatography (HPLC) or UV spectrophotometry. The composition of most antibiotics is generally not limited to a single compound, but rather, a mixture containing one or two major compounds and other congeners (related compounds and impurities). A highly pure antibiotic may not necessarily be highly potent.
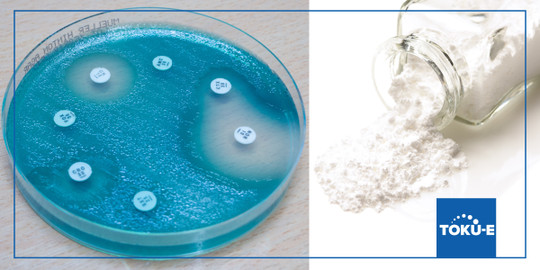
Potency is a measure of the efficacy of the antibiotic (ie how active it is). Potency is measured using microbiological assays, chemical assays (ie HPLC), immunological assays (ie fluorescence polarization immune assay, fluorescence immunoassay), and radioimmunoassay. All microbiological assays involve either diffusion of the antibiotic in the agar or dilution of the antibiotic in agar or broth. Due to the nature of potency testing, impurities may influence the potency of an antibiotic.
Potency may be demonstrated by its inhibitory effect on microorganisms. A reduction in antimicrobial activity will also reveal subtle changes not seen by chemical methods. Therefore, microbial assays remain the gold standard for resolving doubt with respect to potency. Two general methods are used., according to the United States Pharmacopeia <81>: 1) Cylinder-Plate assay and 2) Turbidimetric assay. For the first method, the antibiotic solution is placed in a vertical cylinder and it diffuses through a solid agar layer in a petri plate. This prevents the growth of the added microorganism, and you will see a circular area (‘zone’) around the cylinder. An antibiotic of known potency is used in various concentrations to form a standard curve plated with the concentration plotted on the x-axis and zone of inhibition size plotted on the y-axis. The antibiotic to be tested is compared against the standard curve to determine its potency. For the second method (Turbidimetric Assay), the antibiotic is placed in a fluid medium with the microbial culture and its growth inhibition is monitored. Assay tubes (glass or plastic) are placed in the spectrophotometer.
Potency is measured in either ‘units’ or ‘μg’ of activity. The activity is established by the federal master standard for that particular antibiotic. For example, the USP reference standard is calibrated in terms of the master standard. The USP reference standards for antibiotic substances are held and distributed by the U.S. Pharmacopeial Convention, Inc.
References
https://washingtonmonthly.com/2013/09/25/purity-vs-potency-an-important-distinction-in-drug-policy/