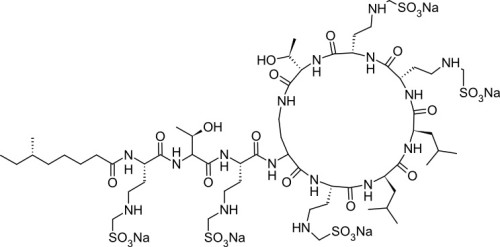
Colistin sulfate, EP (syn: polymyxin E) is a cationic, cyclic polypeptide antibiotic belonging to the polymyxin family. It is a natural product discovered in 1949 that is nonribosomally synthesized by Bacillus polymyxa subspecies colistinus Koyama. Colistin is mixture of Colistin A, B, and C. Colistin targets the bacterial cell membrane, leading to reduced permeability and cell death, and is effective against Gram-negative bacteria. Colistin sulfate, EP is soluble in water (50 mg/ml).
Colistin sulfate, EP, conforms to European Pharmacopoeia specifications.
We also offer:
This product is considered a dangerous good. Quantities above 1 g may be subject to additional shipping fees. Please contact us for details.
| Mechanism of Action | Colistin Sulfate displaces essential ions (magnesium and calcium) in the lipopolysaccharide layer of the cell wall leading to local disturbance of the outer membrane, which causes increased permeability and eventually cell lysis. Colistin also has anti-endotoxin activity. The endotoxin of Gram-negative bacteria is the lipid A portion of the lipopolysaccharide molecules, and colistin binds and neutralizes the lipolysaccharide (Falagas et al, 2005). |
| Spectrum | Colistin Sulfate is used primarily against Gram-negative bacteria. |
| Microbiology Applications | Colistin is commonly used in clinical in vitro microbiological antimicrobial susceptibility tests (panels, discs, and MIC strips) against gram negative microbial isolates. Colistin has also shown high potency against high-resistant superbug strains. Medical microbiologists use AST results to recommend antibiotic treatment options for infected patients. Representative MIC values include:
Media SupplementColistin is routinely used as a selection agent in several types of isolation media: Columbia Blood Agar - Campylobacter selective supplement (Butzler) Columbia Blood Agar - Staphylococcus/Streptococcus selective supplement Columbia Blood Agar - Streptococcus Selective Supplement Listeria Selective Agar - Listeria Selective Supplement Listeria Selective Agar - Modified Listeria Selective Supplement |
| Plant Biology Applications | Plasmodiophora brassicae spores were treated with Colistin Sulfate and used to inoculate hairy roots of cabbage, Chinese cabbage, turnip, and rape in a useful dual culture system for P. brassicae (Asano et al, 1999). |
| Molecular Formula | C53H100N16O13 • xH2SO4 (Colistin Sulfate A) |
| References | Asano T, Kageyama K and Myakumachi (1999) Surface disinfestation of resting spores of Plasmodiophora brassicae used to infect hairy roots of Brassica spp. Phytopathol. 89(4):314-319 PMID 18944777 Falagas ME and Kasiakou SK and Saravolatz LD(2005) Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin. Infect. Dis. 40(9):1333-1341 PMID 15825037 Koen N, van Breda SV and Loots DT (2018) Elucidating the antimicrobial mechanisms of Colistin Sulfate on Mycobacterium tuberculosis using metabolomics. Tuberculosis 1111:14-19 PMID 30029899 Komura S, Kurahashi K (1979) Partial purification and properties of L-2,4-diaminobutyric acid activating enzyme from a polymyxin E producing organism. J. Biochem (Tokyo) 86:1013-1021 Leifert C, Ritchie JY and Waites WM (1991) Contaminants of plant-tissue and cell cultures. World J. Microbiol. Biotechnol. 74):452-469 PMID 24425131 Mueller MJ, Brodschelm W, Spannagl E and Zenk MH (1993) Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc. Natl. Acad. Sci. USA Vol. 90(16):7490-7494 PMID 11607420 Wallace, SJ et al (2012) Interaction of Colistin and colistin methanesulfonate with liposomes: colloidal aspects and implications for formulation J. Pharma Sci 101 (9):3347-3359 PMID 22623044 |