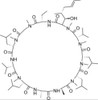
Cyclosporin A, EvoPure is a neutral, cyclic oligopeptide with immunosuppressive activity. Cyclosporin A has the most potent immunosuppressive activity of the metabolites (Cyclosporin B, C, D, E, and H). Cyclosporin A has been used to prevent organ transplant rejection. Cyclosporin A is soluble in ethanol and DMSO.
EvoPure products have been fully characterized by spectral analysis and are shipped with a comprehensive certificate of analysis containing lot-specific HPLC, MS, HNMR, and FTIR data.
For more Cyclosporin products, click here.
| Mechanism of Action | After entering a T-cell, Cyclosporin A associates with the cytosolic protein cyclophilin which helps in protein folding. Cyclosporin A binds to cyclophilins and this complex binds another cytosolic protein phosphatase called Calcineurin (protein phosphatase 2B) that dephosphorylates a transcription factor (nuclear factor of activated T-cells, or NF-AT) needed for expression of interleukin 2 (IL-2.). It also blocks the pathway to nitric oxide synthesis via tumor necrosis factor (TNFa) and Interleukin 1a. |
| Eukaryotic Cell Culture Applications |
Cyclosporins have used as tools to study complex biological networks and pathways, involving protein function, and protein-protein interactions. Cyclosporin A had a suppressive effect on the Hepatitis C virus (HCV) replicon at the RNA level and HCV protein expression in cultured hepatocytes. Cyclosporin A also inhibited multiplication of the HCV genome in a human hepatocyte cell line infected with HCV using HCV-positive plasma (Watashi et al, 2003). Cyclosporin A has been used to establish Epstein-Barr virus-transformed human lymphoblastoid cell lines (Anderson and Gusella 1984). Cyclosporin’s immunosuppressive properties and potential toxicity can be studied during in vitro assays. Other metabolites of Cyclosporin A (AM1, AM1c, DihydroAM1, AM19, and AM4N) can also be studied (Vollenbroeker B et al, 2005). |
| Molecular Formula | C62H111N11O12 |
| References |
Anderson MA and Gusella JF (1984) Use of Cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro 20(11):856-858. PMID 6519667 Matsuda S and Koyasu S (2000) Mechanisms of action of Cyclosporine. Immunopharmacol. 47(2-3): 119-125. PMID 10878286 |