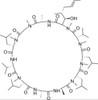
Cyclosporin B, EvoPure is the dihydroxylated metabolite of Cyclosporin A. Cyclosporin B (M-26) and other Cyclosporin metabolites have been found to have lower (<10%) immunosuppressive activity compared Cyclosporin A. Cyclosporin B and other metabolites have been isolated and characterized but have not been extensively studied.
EvoPure products have been fully characterized by spectral analysis and are shipped with a comprehensive certificate of analysis containing lot-specific HPLC, MS, HNMR, and FTIR data.
For more Cyclosporin products, click here.
| Mechanism of Action | Cyclosporin B (and other Cyclosporin A metabolites) have lower immunosuppressive activity but likely operate under the same mechanism as Cyclosporin A described below. After entering a T-cell, Cyclosporin A associates with the cytosolic protein cyclophilin which helps in protein folding. Cyclosporin A binds to cyclophilins and this complex binds another cytosolic protein phosphatase called Calcineurin (protein phosphatase 2B) that dephosphorylates a transcription factor (nuclear factor of activated T-cells, or NF-AT) needed for expression of interleukin 2 (IL-2.). It also blocks the pathway to nitric oxide synthesis via tumor necrosis factor (TNFa) and Interleukin 1a. Cyclosporin A (CsA) immunosuppressive activity stems from its ability to prevent T-cell activation by blocking specific cytokine transcription genes. After entering a T-cell, cyclosporin A (CsA) associates with ubiquitous cytosolic proteins called cyclophilins which aid in protein folding. Cyclosporin A (CsA) : cyclophilin complexes together bind calcineurin, (another cytosolic protein) effectively blocking the pathway to IL-2 gene transcription and T-cell activation. |
| Eukaryotic Cell Culture Applications | Cyclosporins have used as tools to study complex biological networks and pathways, involving protein function, and protein-protein interactions. |
| Cancer Applications | Cyclosporin’s immunosuppressive properties and potential toxicity can be studied during in vitro assays. Other metabolites of Cyclosporin A (AM1, AM1c, DihydroAM1, AM19, and AM4N) can also be studied (Vollenbroeker B et al, 2005). |
| Molecular Formula | C61H109N11O12 |
| References | Anderson MA and Gusella JF (1984) Use of Cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro 20(11):856-858. PMID 6519667 Copelan KR, Yatscoff RW and McKenna RM (1990) Immunosuppressive activity of Cyclosporine metabolites compared and characterized by mass spectrometry and nuclear magnetic resonance. Clin. Chem. 36(2): 225-229. PMID 2137384 Dreyfuss, M et al (1976) Cyclosporin A and C. Eur. J. Appl Microbiol. 3(2): 125-133 Laupacis A et al PA (1982) Cyclosporin A: A powerful immunosuppressant. Can. Med Assoc. J 126(9):1041-1046 PMID 7074504 Matsuda S and Koyasu S (2000) Mechanisms of action of Cyclosporine. Immunopharmacol. 47(2-3): 119-125. PMID 10878286 Matsuda S (2000) Mechanisms of action of cyclosporine. Immunopharmacol. 47(2-3):119-125. PMID 10878286 Oliyai R and Stella VJ (1992) Kinetics and mechanism of isomerization of Cyclosporin A. Pharm. Res. 9(5):617-622 Stiller, CR and Ulan RA (1981) Cyclosporin A: A powerful immunosuppressant."Can. Med. Assn. 126 (1981): 1041-046. Vollenbroeker B et al (2005) Determination of Cyclosporine and its metabolites in blood via HPLC-MS and correlation to clinically important parameters. Transplant Proc. 37(4):1741-1744 PMID 15919451 Wang, PC et al (1989) Isolation of 10 Cyclosporine metabolites from human bile. Drug Metab. Dispos. 17(3): 292-296 PMID 2568911 Watashi K, Hijikata M, Hosaka M, Yamaji M and Shimotohno K (2003) Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatol. 38(5):1282-1288. PMID 14578868 Zheng XS, Chan T, and Zhou HH (2004) Genetic and genomic approaches to identify and study the targets of bioactive small molecules. Chem and Biol 11(5):609-618 PMID 15157872 |