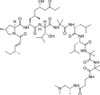
Leucinostatin A is the major component of an atypical nonapeptide complex produced by Paecilomyces lilacinus, first reported in 1973. Leucinostatins display broad bioactivity against Gram-positive bacteria, fungi, plants and tumor cell lines. Leucinostatin A is potentiated by inhibitors such as venturicidin and oligomycin. More recently, interest in Leucinostatin has focused on understanding its activity as an insulin-like growth factor I regulator, an ionophore, inhibitor of cell surface expression of viral glycoproteins and its anti-trypanosomal activity.
Leucinostatin A is soluble in ethanol, methanol, DMF and DMSO. Limited water solubility.
| Mechanism of Action | Leucinostatin A inhibits respiration by uncoupling oxidative phosphorylation. |
| Molecular Formula | C62H111N11O13 |
| References | Arai T et al (1976) A new antibiotic, Leucinostatin, derived from Penicillium lilacinum. J. Antibiot. 26:157 Mori Y et al (1982) Isolation of Leucinostatin A and one of its constituents, the new amino acid, 4-methyl-6-(2-oxobutyl)-2-piperidinecarboxylic acid, from Paecilomyces lilacinus A-267. J. Antibiot. 35:543 Shima A, Fukushima K, Arai T and Terada H (1990) Dual inhibitory effects of the peptide antibiotics Leucinostatins on oxidative phosphorylation in mitochondria. Cell Struct. Funct. 15(1):53-58 PMID 2140298 Kawada M et al (2010) Leucinostatin A inhibits prostate cancer growth through reduction of insulin-like growth factor-I expression in prostate stromal cells. Int. J. Cancer 126:810-818 PMID 19795463 |