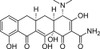
Sancycline is a rare semi-synthetic tetracycline prepared by hydrogenolysis of the chloro and benzylic hydroxy moieties of declomycin, first reported in 1962. As the simplest of the early tetracyclines, sancycline was the first to be totally synthesised by Conover and co-workers.
Sancycline is soluble in ethanol, methanol, DMF and DMSO.
Sancycline is soluble in ethanol, methanol, DMF and DMSO.
| Mechanism of Action | Like other tetracyclines, sancycline acts by reversibly binding to the 30S ribosomal subunit and inhibiting protein translation by blocking entry of aminoacyl-tRNA into the ribosome A site. |
| Molecular Formula | C21H22N2O7 |
| References | Process for the catalytic reduction of 6-hydroxynaphthacenes. McCormich J.R.D. & Jensen E.R. 1962 US Patent 3,019,260. The total synthesis of 6-demethyl-6-deoxytetracycline. Conover L.H. et al. J. Am. Chem. Soc. 1962, 84, 3222. |