
EvoPure is a product line developed by the TOKU-E R&D team which employs proprietary chemical separation methods to produce highly purified, single antimicrobial congeners, metabolites, and impurities, most >99% pure. The composition of most USP and EP pharmaceutical products is generally not limited to a single compound, but rather, a mixture containing one or two major compounds and other congeners (related compounds and impurities). EvoPure products can be used in several in vitro applications:
Analytical reference standards
Upstream pharmaceutical product manufacturing
Gene selection - microbiology, eukaryotic cell biology, plant biology
Toxicity studies
All EvoPure analytical reference standards are manufactured at the TOKU-E purification lab in Ghent, Belgium and have been fully characterized by spectral analysis - HNMR, FTIR, MS, and HPLC. If your project requires compounds that are not currently in the EvoPure product line, please contact us.
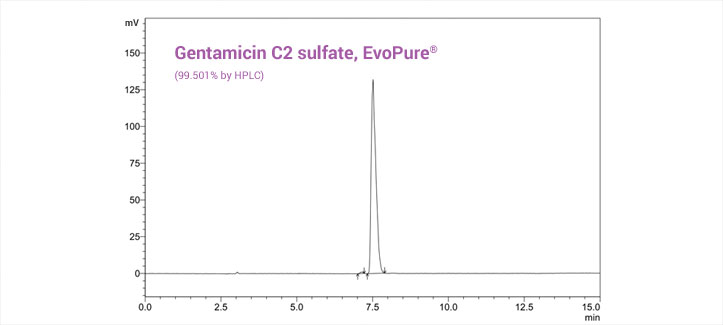
Every EvoPure product is shipped with a comprehensive certificate of analysis which includes product specifications and lot-specific HPLC, mass spec, HNMR, and FTIR data.