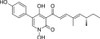
Tenellin is a yellow pigment produced by species of the genus Beauveria, isolated and reported by Canadian researchers in 1968. Structure elucidation in 1977 showed that Tenellin belongs to the rare 4-hydroxypyridone class containing a dienone side chain. Little has been published on the biological activity of Tenellin despite the extensive use of Beauveria species, capable of producing Tenellin, as biocontrol agents for agrochemical pests.
Tenellin is soluble in ethanol, methanol, DMF and DMSO.
| Eukaryotic Cell Culture |
Tenellin inhibited total erythrocyte membrane ATPase activity in a dose-dependent way by as much as 50% at 200 μg/ml. They inhibited Ca2+ATPases to a greater extent than Na+K+-ATPase activity. This inhibitory activity was probably a consequence of membrane disruption (Jeffs and Khachatourians, 1997). Tenellin from Beauveria neobassiana was evaluated for cytotoxicity on biofilm formation by Staphylococcus aureus. Biofilms play a role during chronic infection and reduce antibiotic effectiveness. Teneillin was also evaluated for its ability to inhibit S. aureus biofilm formation. This indicates that Tenellin could be used as a scaffold to create other useful compounds to overcome multi-drug resistant human pathogens (Toche et al, 2024). Tenellin was examined for cytotoxic activity against fibroblast (L929) and ovary (KB3.1) cell lines and was most potent with IC50 of 0.79 μM for both cell types (Toche et al, 2024). |
| Cancer Applications |
Cytotoxicity against breast cancer (MCF-7) and lung cancer (A549) cell lines were evaluated with IC50 of 2 and 0.24 uM respectively (Toche et al, 2024). |
| Insect Biology |
Beauveria species are used in agriculture as biocontrol agents. Beauveria basssiana is used as an eco-friendly alternative to chemical insecticides. It produces many secondary metabolites that act as antimicrobial agents. Eight secondary metabolites including Tenellin (along with bassianin, bassianolide, beauvericin, beauveriolide I, enniatin (A, A1, B) were extracted. Metabolites were analyzed using ultra-performance liquid chromatography-quadrupole-Orbitrap mass spectrometry (UPLC-Q-Orbitrap MS). This method can detect insecticidal fungal secondary metabolites (Kim et al, 2024). |
| References |
El Basyouni SH et al (1968) Pigments of the genus Beauveria. Can. J. Bot. 46:441 Jeffs LB and Khachatourians GG (1997) Toxic properties of Beauveria pigments on erythrocyte membranes. Toxicon 35(8):Pages 1351-1356 Jessena HJ and Gademann K (2010) 4-Hydroxy-2-pyridone alkaloids: Structures and synthetic approaches. Nat. Prod. Rep. 27:1168 Kim JC et al (2024) Rapid analysis of insecticidal metabolites from the entomopathogenic fungus Beauveria bassiana 331R using UPLC-Q-Orbitrap MS. Mycotoxin Res 40:123–132 Toshe R et al (2024) Bioprospection of Tenellins produced by the entomopathogenic fungus Beauveria neobassiana. J. Fungi 10:69 |