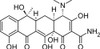
Tetracycline Hydrochloride, EP/USP (syn. Tetracycline HCl) is a light-sensitive bacteriostatic polyketide antibiotic frequently used in a wide range of in vitro cell culture applications. Tetracycline (achromycin) is a first-generation tetracycline antibiotic and was first identified in 1953 by Lloyd Conover’s chemistry team at Pfizer, in collaboration with R.B. Woodward of Harvard University. Tetracycline is a naturally occurring antibiotic from S. aureofaciens, S. rimosus, and S. viridofacien that shows wide-ranging activity against both Gram-negative and Gram-positive bacteria.
Tetracycline is a protein synthesis inhibitor. Tetracycline bind the 30s ribosomal subunit, preventing the aminoacyl-tRNA from attaching to the A site. Consequently, protein synthesis is inhibited. Resistance to tetracycline arises from loss of cell wall permeability, tetracycline efflux, ribosome protection and Tetracycline modification.
Tetracycline is used to study transcriptional activation. Knowledge of Tetracycline led to the development of a popular inducible expression system in eukaryotic cells known as Tet-Off and Tet-On. Tetracycline is also used in multidrug resistance studies and in cell culture applications as a selective agent. Additionally, it promotes expression of the P450 proteins.
Tetracycline Hydrochloride, EP/USP is slightly soluble in aqueous solution.
Tetracycline Hydrochloride, EP/USP conforms to both EP and USP specifications.
We also offer:
| Mechanism of Action | Tetracycline HCl inhibits bacterial growth in Gram-positive and Gram-negative bacteria by disrupting codon-anticodon interactions at the ribosome, thus blocking protein synthesis. Specifically, tetracycline binds to a single site on the 30S ribosomal subunit and inhibit protein synthesis by blocking the attachment of charged aminoacyl-tRNA to the A site on the ribosome. Thus, they prevent introduction of new amino acids to the nascent peptide chain.
Mammalian cells are not vulnerable to the effect of Tetracycline as these cells contain no 30S ribosomal subunits so no accumulation results. |
| Spectrum | Tetracycline HCl is a broad-spectrum antibiotic, effective against both Gram-positive, Gram-negative bacteria, and mycoplasma. A few species of bacteria display intrinsic resistance to tetracycline, including Pseudomonas aeruginosa. Acquired (as opposed to inherent) resistance has proliferated in many pathogenic organisms and greatly eroded the versatility of Tetracycline derivatives. Resistance amongst Staphylococcus, Streptococcus, Neisseria gonorrhoeae, and members of the Enterobacteriaceae is now quite common. Tetracyclines show activity against protozoan parasites as well. |
| Impurity Profile | 4-Epitetracycline: Not more than 3.0% Anhydrotetracycline: Not more than 0.5% 4-Epianhydrotetracycline: Not more than 0.5% 2-Acetyl-2-decarbamoyl-tetracycline: Not more than 1.5% Heavy Metals: Not more than 50 ppm Sulphate Ash: Not more than 0.5% Bacterial Endotoxin: Not more than 0.5 IU/mg |
| Microbiology Applications | Tetracycline HCl is routinely used as a selective agent to select for bacterial cells that have been transformed with a plasmid that contains the tetracycline resistance gene, tet. Tetracycline is typically used at 10 µg/mL. Tetracycline HCl has activity against the bacteria causing: Q fever, Rocky Mountain spotted fever, tick fevers, typhus fever, and Brill-Zinsser disease. It is also used to to treat upper respiratory infections and acne. It has been used in studies of multidrug resistance and potential side effects including acute pancreatitis. It has been recognized for some time that the spectrum of activity of Tetracyclines encompasses various protozoan parasites such as P. falciparum, Entamoeba histolytica, Giardia lamblia, Leishmania major, Trichomonas vaginalis, and Toxoplasma gondii. |
| Plant Biology Applications | Tetracycline has been shown to suppress symptoms of aster yellows disease in China Aster and Chrysanthemum plants. Tetracyclines are sprayed onto fruit trees and other plants to treat infection by Erwinia amylovora, injected into palm trees to treat mycoplasma infections (lethal yellow), and used to control infection of seeds by Xanthomonas campestris (black rot). Multiple applications of tetracycline resulted in symptomless axillary plant growth (Davis R.E. and Whitcomb, 1970). |
| Eukaryotic Cell Culture Applications | Tetracycline is routinely used to select for cells containing resistance plasmids in cell lines such as HeLa at an effective concentration of 1 µg/mL. Tetracycline is used to study transcriptional activation. Knowledge of Tetracycline led to the development of a popular inducible expression system in eukaryotic cells known as Tet-Off and Tet-On. This system has the advantages of being a conditional system that is both reversible and tightly controlled with a lower incidence of leaky (background) expression compared to other inducible systems. Tet-Off systems are also used in generating transgenic mice, which conditionally express a gene of interest. Since the 19bp TetO sequence is naturally absent from mammalian cells, pleiotropy is minimized compared to hormonal control used by other inducible expression systems. Today there are several popular systems including Tet-off, Tet-on and Tet autoregulatory systems which help to minimize leaky background in uninduced cells. When using the Tet system in mammalian cell culture, it is important to either use animal-free media or to test each batch of fetal bovine serum (FBS) to confirm that contaminating tetracyclines are absent or are too low to interfere with induction. Doxycycline (Dox) is a water-soluble tetracycline derivative that is preferred for almost all Tet-controlled gene expression systems. Standard concentration for cell culture applications is 10mg/L, for additional information on your cell culture needs, please visit our cell-culture database. |
| Cancer Applications | Tetracycline derivatives induce apoptosis in osteoclasts, Jurkat T lymphocyte cells and in cultured monocytes and macrophages. It is a compound that has been shown to induce apoptosis in various cells.
Tetracycline HCl and chemically modified tetracyclines, like Col-3, inhibit the activity of several matrix metalloproteinases (MMPs) that are important enzymes in tumor cell invasion and metastatic ability. |
| Insect Biology Applications | Tetracyclines have applications in the treatment of insects of commercial value; e.g., oxytetracycline is used to treat foulbrood disease of the honeybee, which is caused by either Bacillus larvae or Streptococcus pluton. |
| Molecular Formula | C22H24N2O8 • HCl |
| Residual Solvents | 1-Butanol: Not more than 5000 ppm Acetone: Not more than 5000 ppm |
| References |
Backman K, Boyer HW (1983) Tetracycline resistance in Esherichia coli is mediated by one polypeptide. Gene 26(2-3): 197-203 PMID 6323260 Chopra I and Roberts M (2001) Tetracycline Antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Molec. Biol. Rev. 65(2):232-60 PMID 11381101 Davis RE and Whitcomb RF (1970) Evidence on possible Mycoplasma etiology of Aster Yellows disease. Infect. Immun. Infect. Immun. 2(2):201-208 PMID 16557820 Gatz C (1995) Novel inducible/repressible gene expression systems. Methods Cell Biol. 50: 411-424 PMID 8531813 Green M and Sambrook J. Molecular Cloning: A Laboratory Manual 4th ed. Vol II, Cold Spring Harbor Laboratory Press, New York |
| MIC | Bifidobacterium adolescentis| 15.62|| Bifidobacterium animalis| 3.95|| Bifidobacterium bifidum| 15.6 - 31.25|| Bifidobacterium breve| 31.25|| Bifidobacterium infantis| 125|| Bifidobacterium longum| 31.25 - 62.5|| Bifidobacterium pseudolongum| 15.62|| Bifidobacterium sp.| 7.81 - 31.25|| Bifidobacterium thermophilum| 62.5|| Brucella suis| 125|| Escherichia coli| 1 - >128|| Lactobacillus acidophilus| 0.98 - 125|| Lactobacillus bulgaricus| 15.6 - 125|| Lactobacillus casei| 0.98 - 125|| Lactobacillus lactis| 7.8 - 125|| Lactobacillus paracasei| 0.98|| Lactobacillus plantarum| 7.8 - 125|| Lactobacillus rhamnosus| 7.8 - 250|| Prevotella intermedia| 0.09 - 0.1|| Shigella spp.| 1 - 128|| |