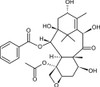
10-Deacetyl Baccatin III (syn: 10-DAB) is a natural organic compound produced by the Pacific Yew tree. It is used as a key backbone intermediate for the semi-synthesis of Paclitaxel (Taxol), an anti-cancer drug and Docetaxel and their analogs. It is used to study the biosynthetic pathway of Taxol. The trunk and leaves contain the highest concentration of 10-Deacetyl Baccatin III, but it may be found in parts of the Yew tree.
| Mechanism of Action | Paclitaxel (Taxol) has a unique mode of action involving abnormal polymerization of tubulin and disruption of mitosis. |
| Plant Biology Applications | Taxus cell culture may be an alternate source of Paclitaxel, and significantly increased amounts of Paclitaxel were observed after exposure to methyl jasmonate (Yukimune et al, 1996). |
| Cancer Applications | 10-Deacetyl Baccatin III is commonly used as an intermediate in the synthesis of paclitaxel (Taxol), an anti-cancer drug. |
| Solubility | Soluble in methanol. Insoluble in water and ethanol. |
| References |
Gelderblom H et al (1999) Disposition of [G-(3)H]paclitaxel and cremophor EL in a patient with severely impaired renal function. Drug Metab Dispos. 27(11):1300-1305 PMID 10534315 Gueritte-Voegelein F, Senih V, David B, Guenard D and Potier P (1986) Chemical studies of 10-Deacetyl Baccatin III: Hemisynthesis of taxol derivatives. Tetrahed. 42(16):4451-4460 Kant J et al (1994) A chemoselective approach to functionalize the C-10 position of 10-Deacetylbaccatin III. Synthesis and biological properties of novel C-10 Taxol® analogues. Tetrahed. Lett. 35(31):5543-5546 Ojima I et al (1997) Syntheses and structure-activity relationships of toxoids derived from 14 beta-hydroxy-10-deacetylbaccatin III. J. Med. Chem. 40(3):267-278 PMID 9022793 Yukimune Y, Tabata H, Higashi Y and Hara Y (1996) Methyl jasmonate-induced overproduction of paclitaxel and Baccatin III in Taxus cell suspension cultures. Nature Biotechnol. 14(9):1129-1132 |