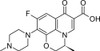
Levofloxacin is a broad-spectrum third-generation fluoroquinolone and one of the isomers of ofloxacin, specifically the optical S-(-) isomer. It is a DNA gyrase inhibitor shown to inhibit topoisomerase IV. Levofloxacin was synthesized by scientists at Daiichi Pharmaceutical Co. Ltd (Tokyo, Japan) and is one of the later generation fluoroquinolones referred to as 'respiratory quinolones' to distinguish them from earlier fluoroquinolones which had modest activity towards Streptococcus pneumoniae. It acts as a bactericide. Levofloxacin is called a chiral switch (a chiral compound that has been approved as a racemate but has been re-developed as a single enantiomer. It can be used ot study antibiotic resistance.
Levofloxacin is soluble in glacial acetic acid and chloroform. It is insoluble in water.
| Mechanism of Action |
Fluoroquinolone antibiotics target bacterial DNA gyrase (a type IIA topoisomerase) an enzyme which reduces DNA strain during replication. Because DNA gyrase is required during DNA replication, subsequent DNA synthesis and ultimately cell division is inhibited. It has also been shown to inhibit topoisomerase IV, which is another type IIA topoisomerase. Topoisomerase IV is needed to separate DNA that has been replicated prior to bacterial cell division. If DNA remains unseparated, the process stops and the bacterial cell division ceases. Authors found anti-cancer activity was due to inhibition of mitochondrial electron transport chain complex I and III, leading to mitochondrial respiration inhibition, and reduction of ATP production. Additionally, it increased levels of reactive oxygen species (ROS), mitochondria superoxide and hydrogen peroxide in vitro. |
| Spectrum |
Levofloxacin is a broad-spectrum antibiotic targeting most aerobic Gram-positive and Gram-negative bacteria. It is moderately active against anaerobes. It can also be used against Mycoplasmas. It is effective against pathogens causing pneumonia such as Streptococcus pneumoniae, and Haemophilus influenzae. Resistance to fluoroquinolones is common in Staphylococcus and Pseudomonas. Resistance can occur in multiple ways, one of which involves an alteration in topoisomerase IV enzyme. |
| Cancer Applications |
Levofloxacin was active against a panel of lung cancer cell lines via inhibition of proliferation and inducing apoptosis, in vitro. This is novel research to show that it was active against multiple lung cancer cell lines, and attributed to its ability to induce mitochondrial dysfunctions and oxidative stress (Song et al, 2016). Levofloxacin was active against breast cancer cell lines in vitro, acting synergistically with 5-Fluorouracil in breast cancer. It acts via mitochondrial biogenesis inhibition, and authors showed the effects were reversed by acetyl-L-carnitine (ALCAR, a mitochondrial fuel) confirmation that its action is via inhibition of mitochondrial biogenesis. Breast cancer cells have increased mitocondrial biogenesis compared to normal cells, and thus targeting mitochondrial biogenesis has been a potential anti-cancer strategy. |
| Impurity Profile | Impurity A| (-)-(S)-9-Fluoro-2,3-dihydro-3-methyl-10-(1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid; N-Desmethyl Levofloxacin|117707-40-1|C17H18FN3O4|347.34| Impurity B| 8,9-Difluoro-3-methyl-6-oxo-2,3-dihydro-6H-1-oxa-3a-aza-phenalene-5-carboxylic acid; Levofloxacin Difluoro Carboxylic Acid|100986-89-8|C13H9F2NO4|281.21| Impurity C| Ethyl (S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylate ; Levofloxacin Ethyl Ester|177472-30-9|C20H24FN3O4|389.42| Impurity D||||| Impurity UN1| LEVOFLOXACIN N-OXIDE ; (-)-(S)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid N-oxide||C18H20FN3O5|377.37| Levofloxacin R-Isomer| (+)-(R)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid; (R)-(+)-Ofloxacin; D-Ofloxacin; D-Levofloxacin|100986-86-5|C18H20FN3O4|361.37| Levofloxacin Desethylene Impurity| N,N*-Desethylene Levofloxacin Hydrochloride; (-)-(S)-9-Fluoro-2,3-dihydro-3-methyl-10-[(2-methylamino)ethylamino]-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine||C16H19ClFN3O4|371.79| Levofloxacin Descarboxy Impurity| (-)-(S)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine|178964-53-9|C17H20FN3O2|317.36| Levofloxacin Desethylene Diformyl Impurity| Desethylene Diformyl Levofloxacin; N,N*-Desethylene-N,N*-diformyl Levofloxacin; (S)-9-Fluoro-10-[formyl[2-(formylmethylamino)ethyl]amino]-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid|151377-74-1|C18H18FN3O6|391.35| Levofloxacin Desfluoro Impurity| (-)-(S)-2,3-Dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid|117620-85-6|C18H21N3O4|343.38| |
| Microbiology Applications | Levofloxacin is commonly used in clinical in vitro microbiological antimicrobial susceptibility tests (panels, discs, and MIC strips) against Gram-positive and Gram-negative microbial isolates. Medical microbiologists use AST results to recommend antibiotic treatment options. Representative MIC values include:
Resistance to fluoroquinolones is common in Staphyloccus and Pseudomonas. Resistance occurs in multiple ways, one way is via alteration in topoisomerase IV enzyme. Microfluidic droplet systems are popular analytical methods used in antimicrobial susceptibility testing (AST) to help study antimicrobial resistance (AMR). Droplet methods have many advantages over conventional AST methods. Authors used several antibiotics from TOKU-E including Levofloxacin to review the physicochemical properties to predict retention of antibiotics in droplets (Ruszczak et al, 2023). |
| Molecular Formula | C18H20FN3O4 • 0.5H2O |
| Solubility | Chloroform: Soluble Glacial acetic acid: Soluble Water: Insoluble |
| References |
Bebear CM, Renaudin H, Bryskier A, Bebear C (2000) Comparative activities of telithromycin (HMR 3647), levofloxacin, and other Antimicrobial Agents against human Mycoplasmas . Antimicrob. Agents and Chemother. 44 (7) 1980-1982 PMID 10858366 Davis R and Bryson HM (1994) Levofloxacin. A review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs. 47(4):677-700 PMID 7516863 Ruszczak A, Jankowski P, Vasantham S, Scheler O and Garstiecki P (2023) Physicochemical properties predict retention of antibiotics in water-in-oil droplets. Anal. Chem. 95(2): 1574−1581 PMID 36598882 Song et al (2016) Antibiotic drug Levofloxacin inhibits proliferation and induces apoptosis of lung cancer cells through inducing mitochondrial dysfunction and oxidative damager. Biomed. Pharmacother. 84:1137-1143 Staff (2009) New FDA requirements for post-marketing studies and clinical trials: Patent Strategy . memoANDA. Fish and Richardson pVIII. Archived from the original on 27Aug 2025. US 5053407. Wolfson JS and Hooper DC (1985) The fluoroquinolones: Structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob. Agents Chemother. 28(4):581-586 PMID 3000292 Yu M, Li R and Zhang J (2016) Repositioning of antibiotic Levofloxacin as a mitochondrial biogenesis inhibitor to target breast cancer. Biochem. Biophys. Res. Comm. 471(4):639-645 |
| MIC | Aeromonas spp.| 0.0625|| Alcaligenes faecalis| 0.39 - 25|| Bacillus circulans| 0.25 - 8|| Bacillus subtilis (ATCC 6051)| 6.25|| Bacteroides capillosus | ≤0.06 - >8|| Bacteroides distasonis| 0.5 - 128|| Bacteroides eggerthii| 4|| Bacteroides fragilis| 0.5 - 128|| Bacteroides merdae| 0.25 - >32|| Bacteroides ovatus| 0.25 - 256|| Bacteroides thetaiotaomicron| 1 - 256|| Bacteroides uniformis| 4 - 128|| Bacteroides ureolyticus| ≤0.06 - >8|| Bacteroides vulgatus| 1 - 256|| Bifidobacterium adolescentis| 0.25 - >32|| Bifidobacterium bifidum | 8|| Bifidobacterium breve| 0.25 - 8|| Bifidobacterium longum| 0.25 - 8|| Bifidobacterium pseudolongum| 8|| Bifidobacterium sp.| 0.25 - >32|| Bilophila wadsworthia| 0.25 - 16|| Brevibacterium spp.| 0.12 - 8|| Brucella melitensis| 0.5|| Burkholderia cepacia| 0.25 - 512|| Campylobacter coli| 0.015 - 128|| Campylobacter concisus| ≤0.06 - >8|| Campylobacter gracilis| ≤0.06 - >8|| Campylobacter jejuni| 0.015 - 128|| Campylobacter mucosalis| ≤0.06 - >8|| Campylobacter rectus| ≤0.06 - >8|| Campylobacter showae| ≤0.06 - >8|| Campylobacter spp.| 0.25|| Campylobacter sputorum| ≤0.06 - >8|| Capnocytophaga ochracea | ≤0.06 - >8|| Capnocytophaga spp.| 0.006 - 2|| Chlamydia pneumonia| 0.125 - 1|| Chlamydia psittaci| 0.5|| Chlamydia trachomatis| 0.12 - 1|| Chlamydophila pneumonia| 0.5|| Citrobacter diversus| 0.015 - 0.125|| Citrobacter freundii| ≤0.00625 - >64|| Citrobacter koseri| 0.015 - >8|| Citrobacter spp.| 0.008 - 64|| Clostridium baratii| 0.5 - 4|| Clostridium bifermentans| 0.5 - 4|| Clostridium cadaveris| 0.12 - 16|| Clostridium clostridioforme| 16|| Clostridium difficile| 1 - 128|| Clostridium hastiforme| 0.5 - 4|| Clostridium innocuum| 0.12 - 16|| Clostridium malenominatum| 0.12 - 16|| Clostridium oroticum| 0.12 - 16|| Clostridium paraputrificum (ATCC 25780)| 1|| Clostridium perfringens| 0.12 - 32|| Clostridium putrificum| 0.25|| Clostridium ramosum| 0.5 - 8|| Clostridium septicum| 0.125|| Clostridium sordellii| 0.12 - 16|| Clostridium sporogenes| 0.12 - 16|| Clostridium spp.| 0.06 - 32|| Clostridium tertium| 0.5 - 4|| Collinsella aerofaciens| ≤0.06 - 2|| Corynebacterium afermentans| 0.12 - 8|| Corynebacterium amycolatum| 0.12 - 64|| Corynebacterium jeikeium| 0.12 - 16|| Corynebacterium minutissimum| 0.06 - 16|| Corynebacterium pseudodiphtheriticum| 0.25 - 16|| Corynebacterium spp.| 0.125 - 64|| Corynebacterium striatum| 0.12 - 64|| Corynebacterium urealyticum| 0.12 - >128|| Coryneform| 0.12 - 8|| Desulfovibrio piger | 0.125|| Dialister pneumosintes | ≤0.06 - >8|| Eggerthella lenta| 0.5|| Eikenella corrodens| ≤0.06|| Enterobacter aerogenes| ≤0.008 -64|| Enterobacter agglomerans| ≤0.25 - >8|| Enterobacter cloacae| ≤0.003 - 64|| Enterobacter sakazakii| ≤0.25 - 2|| Enterobacter spp.| <0.004 - 64|| Enterobacteriaceae| ≤0.008 - >16|| Enterococci| 0.5 - 50|| Enterococcus avium| 0.06 - >64|| Enterococcus casseliflavus| 0.06 - >4|| Enterococcus durans| 0.06 - >4|| Enterococcus faecalis| 0.006 - 9750|| Enterococcus faecium| 0.06 - >128|| Enterococcus gallinarum| 0.06 - 32|| Enterococcus hirae| 0.06 - >4|| Enterococcus mundtii| 0.06 - >4|| Enterococcus raffinosus| 0.06 - >4|| Enterococcus spp.| 0.06 - >4|| Erysipelothrix rhusiopathiae| 0.06|| Erysipelothrix spp.| 0.06 - 0.12|| Escherichia coli| ≤0.004 - >128|| Eubacterium| 0.12 - 4|| Eubacterium lentum| ≤0.06 - 2|| Eubacterium limosum| 0.12 - 4|| Eubacterium nodatum| 0.12 - 4|| Eubacterium saburreum| 0.25 - >8|| Eubacterium spp.| ≤0.06 - 8|| Eubacterium timidum| ≤0.06 - 2|| Eubacterium yurii| ≤0.06 - 2|| Finegoldia magna| 0.094 - 128|| Fusobacteria| 0.5 - >32|| Fusobacterium| ≤0.015 - 4|| Fusobacterium mortiferum| 0.5 - 1|| Fusobacterium naviforme| 0.25 - >8|| Fusobacterium necrophorum| 0.25 - >8|| Fusobacterium nucleatum| ≤0.03 - >32|| Fusobacterium spp.| 0.03 - >32|| Fusobacterium varium| 4 - >32|| Gardnerella vaginalis| 0.5 - >8|| Gemella morbillorum| 0.006 - 2|| Gram-Positive Anaerobic Cocci| 0.25 - 8|| Haemophilus influenzae| ≤0.004 - 16|| Haemophilus parainfluenzae| 0.006 - 2|| Haemophilus spp.| 0.32 - 16|| Helicobacter pylori| 0.008 - 250|| Hemolytic streptococci| 0.25 - 2|| Hemophilus influenzae | 0.016 - 0.031|| Klebsiella| ≤0.03 - >8|| Klebsiella oxytoca| 0.015 - 8|| Klebsiella pneumonia| <0.004 - 128|| Klebsiella spp.| 0.015 - 1|| Lactobacillus acidophilus (JCM 1132)| 32|| Lactobacillus brevis (JCM 1059)| 8|| Lactobacillus casei (JCM 1134)| 1|| Lactobacillus catenaforme| 0.25 - 8|| Lactobacillus delbrueckii| 0.25 - 8|| Lactobacillus fermentum (JCM 1173)| 8|| Lactobacillus jensenii| 0.12 - 4|| Lactobacillus oris| 0.25 - 8|| Lactobacillus plantarum| 0.25 - 8|| Lactobacillus reuteri (JCM 1112)| 16|| Lactobacillus salivarius (JCM 1231)| 1|| Lactobacillus spp.| 0.12 - 8|| Legionella adelaidensis| ≥0.008|| Legionella anisa| ≥0.032|| Legionella birminghamiensis| ≥0.032|| Legionella bozemanii| ≥0.023|| Legionella brunensis| ≥0.032|| Legionella cherrii| ≥0.016|| Legionella cincinnatiensis| ≥0.016|| Legionella dumofii| ≥0.025|| Legionella erythra| ≥0.016|| Legionella fairfieldensis| ≥0.025|| Legionella feeleii| ≥0.04|| Legionella geestiana| ≥0.004|| Legionella gormanii| ≥0.023|| Legionella gratiana| ≥0.064|| Legionella hackeliae| ≥0.125|| Legionella israelensis| ≥0.016|| Legionella jamestowniensis| ≥0.032|| Legionella jordanis| ≥0.032|| Legionella lansingensis| 0.008 - 0.125|| Legionella longbeachae| ≥0.027|| Legionella maceachernii| ≥0.016|| Legionella micdadei| ≥0.018|| Legionella moravica| ≥0.008|| Legionella nautarum| ≥0.064|| Legionella oakridgensis| ≥0.016|| Legionella pneumophila| 0.002 - 0.5|| Legionella quateirensis| ≥0.008|| Legionella quinlivanii| ≥0.032|| Legionella rubrilucens| ≥0.016|| Legionella sainthelensi| ≥0.032|| Legionella santicrucis| ≥0.012|| Legionella shakespearei| ≥0.125|| Legionella spiritensis| ≥0.016|| Legionella spp.| 0.0039 - 0.06|| Legionella steigerwaltii| ≥0.024|| Legionella tucsonensis| ≥0.032|| Legionella wadsworthii| ≥0.008|| Legionella worsleiensis| ≥0.006|| Leptotrichia buccalis| 0.25 - >8|| Leuconostoc spp.| 1 - 2|| Leuconostoc spp.| <1000|| Listeria monocytogenes| 0.25 - 2|| Listeria spp.| 0.5 - 2|| Micrococcus luteus (LB 14110)| 200|| Micromonas micros| 0.125 - 0.5|| Moraxella catarrhalis| ≤0.006 - >4|| Moraxella sp.| 0.064|| Morganella morganii| 0.0125 - 64|| Morganella spp.| ≤0.25 - 64|| Mycobacterium abscessus| 1 - 64|| Mycobacterium avium| 0.5 - 64|| Mycobacterium chelonae| 0.25 - 64|| Mycobacterium fortuitum| ≤0.03 - 1|| Mycobacterium intracellulare| 16 - 25|| Mycobacterium marinum| 0.032 - 16|| Mycobacterium mucogenicum| 0.12 - 2|| Mycobacterium peregrinum| ≤0.03 - 0.25|| Mycobacterium tuberculosis| 0.0625 - 8|| Mycoplasma fermentans| ≤0.008 - 1|| Mycoplasma genitalium| 0.5 - 1|| Mycoplasma hominis| 0.016 - 2|| Mycoplasma penetrans| 0.06 - 0.25|| Mycoplasma pneumonia| 0.063 - 2|| Neisseria cinerea| 0.007 - 1|| Neisseria elongata | 0.008 - 1|| Neisseria lactamica| <0.003 - 0.015|| Neisseria meningitidis| ≤0.003 - 0.03|| Neisseria mucosa| 0.007 - 0.03|| Neisseria perflava/sicca| <0.003 - 0.12|| Neisseria polysaccharea| <0.003|| Neisseria sicca| 0.001 - 1|| Nonsporing gram-positive rods| 0.25 - 32|| Oerskovia spp.| 0.12 - 8|| Olsenella uli| 0.25 - 8|| Pantoea agglomerans| 0.015 - 0.06|| Parvimonas micra| 0.125 - 3|| Pasteurella multocida| <0.5|| Pediococcus spp.| 8|| Peptoniphilus asaccharolyticus| 4 - 128|| Peptoniphilus gorbachii | 3 - 64|| Peptoniphilus harei | 2 - 64|| Peptoniphilus indolicus| 4|| Peptoniphilus ivorii | 0.38 - 64|| Peptoniphilus lacrimalis | 3 - 8|| Peptoniphilus octavius | 4|| Peptostreptococcus| ≤0.015 - 16|| Peptostreptococcus anaerobius| 0.12 - 128|| Peptostreptococcus asaccharolyticus| 0.12 - 25|| Peptostreptococcus magnus| 0.12 - 16|| Peptostreptococcus micros| 0.12 - 16|| Peptostreptococcus prevotii| 0.12 - 16|| Peptostreptococcus spp.| 0.12 - 664|| Peptostreptococcus tetradius| 0.25 - 16|| Plesiomonas shigelloides| 0.0625|| Pneumococci| 0.5 - >32|| Porphyromonas| ≤0.015 - 16|| Porphyromonas endodontalis | ≤0.06 - >32|| Porphyromonas gingivalis| ≤0.06 - >8|| Porphyromonas spp.| 0.06 - 2|| Prevotella bivia| ≤0.03 - 16|| Prevotella buccalis| ≤0.06 - >8|| Prevotella corporis | 0.125 - 4|| Prevotella dentalis | ≤0.06 - >8|| Prevotella denticola| 0.125 - 16|| Prevotella disiens| ≤0.06 - 16|| Prevotella heparinolytica | 1|| Prevotella intermedia| ≤0.06 -16|| Prevotella loescheii| 0.125 - 16|| Prevotella melaninogenica| 0.125 - 16|| Prevotella nigrescens| ≤0.06 - >8|| Prevotella oralis| ≤0.06 -16|| Prevotella oris| ≤0.06 - 16|| Prevotella pallens | ≤0.06 - >8|| Prevotella spp.| ≤0.06 - 16|| Prevotella tannerae | 0.125 - 4|| Prevotella zooglioformans | ≤0.06 - >8|| Propionibacteria| 0.25 - 0.5|| Propionibacterium| 0.12 - 8|| Propionibacterium avidum| 0.25 - 8|| Propionibacterium granulosum| 0.25|| Propionibacterium sp. | 0.25 - >32|| Proteus| 0.03 - >128|| Proteus mirabilis| 0.015 - 12220|| Proteus rettgeri| ≤0.06 - >8|| Proteus spp.| 0.25|| Proteus vulgaris| 0.015 - 8|| Providencia alcalifaciens| 0.06 - >16|| Providencia rettgeri| 0.06 - >16|| Providencia spp.| ≤0.25 - 64|| Providencia stuartii| 0.06 - >16|| Pseudomonas aeruginosa| ≤0.008 - 256|| Pseudomonas savastanoi| ≥3.13|| Pseudomonas syringae. pv. tomato| ≥6.25|| Rhodococcus equi| 0.25 - 1|| Rothia dentocariosa| 0.006 - 2|| Rothia mucilaginosa| 0.006 - 2|| Ruminococcus gnavus| 64|| Salmonella enterica| <0.008 - 16|| Salmonella enteritidis| 0.00625 - 0.1|| Salmonella spp.| 0.015 - 2|| Salmonella typhi| <0.008 - 1220|| Salmonella/Shigella spp.| 0.015 - 0.06|| Selenomonas flueggei | ≤0.06 - >8|| Selenomonas infelix | ≤0.06 - >8|| Selenomonas spp.| ≤0.06 - >8|| Serratia liquefaciens| 0.015 - 1|| Serratia marcescens| 0.015 - >128|| Serratia spp.| ≤0.25 - 64|| Shigella boydii| 0.015 - 0.06|| Shigella flexneri| ≤0.015 - 0.06|| Shigella sonnei| ≤0.015 - 0.06|| Shigella spp.| 0.004 - 0.25|| Staphylococci | ≤0.03 - >128|| Staphylococcus aureus| 0.006 - >128|| Staphylococcus auricularis| ≤0.03 - >16|| Staphylococcus capitis| ≤0.03 - 128|| Staphylococcus chromogenes | ≤0.06 - 128|| Staphylococcus cohnii| ≤0.03 - >16|| Staphylococcus epidermidis| 0.006 - >128|| Staphylococcus equorum| 0.06 - >16|| Staphylococcus haemolyticus| ≤0.03 - 128|| Staphylococcus hominis| ≤0.03 - 128|| Staphylococcus lugdunensis| ≤0.03 - >8|| Staphylococcus pneumonia| ≥1|| Staphylococcus saccharolyticus| 0.125 - 0.5|| Staphylococcus saprophyticus| ≤0.03 - 128|| Staphylococcus sciuri| ≤0.03 - 8|| Staphylococcus simulans| ≤0.03 - >4|| Staphylococcus spp.| ≤0.03 - >128|| Staphylococcus warneri| ≤0.03 - >16|| Staphylococcus xylosus| ≤0.03 - >4|| Stenotrophomonas maltophilia| 0.03 - 63|| Streptococci| ≤0.015 - 64|| Streptococcus acidominimus | 0.25 - 2|| Streptococcus agalactiae| ≤0.06 - 32|| Streptococcus anginosus| 0.12 - >8|| Streptococcus bovis| 0.12 - >8|| Streptococcus constellatus| 0.12 - >8|| Streptococcus epidermidis| 0.12 - >16|| Streptococcus intermedius| ≤0.03 - >8|| Streptococcus milleri| 0.25 - 1|| Streptococcus mitis| 0.12 - >8|| Streptococcus oralis| 0.12 - >8|| Streptococcus ovis | 0.25 - 2|| Streptococcus parasanguinis | 0.25 - 2|| Streptococcus pluranimalium | 0.25 - 2|| Streptococcus pneumonia| ≤0.03 - 16000|| Streptococcus pyogenes| 0.06 - 16|| Streptococcus salivarius| 0.12 - >8|| Streptococcus sanguinis| 0.12 - >8|| Streptococcus sobrinus| 0.25 - 2|| Streptococcus spp.| 0.25 - 1|| Streptococcus thoraltensis | 0.25 - 2|| Sutterella wadsworthensis| 0.25 - 32|| Turicella otitidis| 0.12 - 8|| Ureaplasma spp.| 0.125 - 2|| Ureaplasma urealyticum| 0.5 - 1|| |