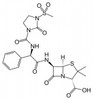
Mezlocillin Sodium is broad-spectrum, semisynthetic, β-lactam antibiotic and ureido penicillin derived from ampicillin. It is sparingly soluble in aqueous solution.
| Mechanism of Action | β-lactams interfere with PBP (penicillin binding protein) activity involved in the final phase of peptidoglycan synthesis. PBP’s are enzymes which catalyze a pentaglycine crosslink between alanine and lysine residues providing additional strength to the cell wall. Without a pentaglycine crosslink, the integrity of the cell wall is severely compromised and ultimately leads to cell lysis and death. Resistance to β-lactams is commonly due to cells containing plasmid-encoded β-lactamases, however, Mezlocillin is resistant to a number of β-lactamases. |
| Spectrum | Mezlocillin is a broad-spectrum antibiotic targeting a wide variety of Gram-positive and Gram-negative bacteria. |
| Microbiology Applications | Mezlocillin is commonly used in clinical in vitro microbiological antimicrobial susceptibility tests (panels, discs, and MIC strips) against Gram-positive and Gram-negative microbial isolates. Medical microbiologists use AST results to recommend antibiotic treatment options. Representative MIC values include:
|
| Eukaryotic Cell Culture Applications | Mezlocillin did not cause any cytotoxicity in cell cultures of mouse sarcoma L-1, human lung cancer E-14, or human malignant melanoma MEW (Roszkowski et al, 1984). |
| Molecular Formula | C21H24N5NaO8S2 |
| References |
Bodey GP and Pan T (1977) Mezlocillin: In vitro studies of a new broad-spectrum penicillin. Antimicrob.Agents Chemother. 11 (1): 74-79 PMID 836016 Guzmán, Flavio, MD (2008) Beta lactam antibiotics (penicillins and Cephalosporins) mechanism of action. Medical Pharmacology. Pharmacology Corner. Pitout JD, Sanders CC, Sanders WE (1997) Antimicrobial resistance with focus on beta-lactam resistance in Gram-negative bacilli. Am J Med 103:51 Roszkowski K, Ko HL, Roszkowski W, Jeljaszewicz J, and Pulverer G. (1984) Effects of cefotaxime, clindamycin, Mezlocillin, and piperacillin on mouse sarcoma L-1 tumor. Cancer Immunol. 18(3):164-168 PMID 6095992 Soares LA and Trabulsi LR (1979) Studies on the antibacterial activity of two new acylureidopenicillins, Mezlocillin and azlocillin. Arzneimittelforschung. 29(12a):1932-1934. PMID 543893 |
| MIC | Bacteroides fragilis| 0.5 - ≥256|| Bacteroides xylanisolvens | 8|| Borrelia burgdorferi S.L.| <0.06 - 1|| Citrobacter diversus| 4 - 128|| Citrobacter freundii| 1 - 64|| Edwardsiella hoshinae | 0.13|| Edwardsiella ictaluri | 0.13 - 1|| Edwardsiella tarda| 0.13 - 4|| Enterobacter cloacae| 2 - 256|| Enterobacteriaceae| 0.25 - 128|| Escherichia coli| 1 - 32|| Helicobacter pylori| 0.125 - 0.25|| Klebsiella pneumonia| 1 - 8|| Morganella morganii| 0.5 - 32|| Proteus vulgaris| 1 - 32|| Pseudomonas spp.| 0.5 - 512|| Serratia marcescens| 2 - 32|| Staphylococci| 0.12 - 128|| |