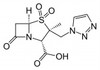
Tazobactam Sodium is the sodium salt of Tazobactam, a penicillanic acid sulfone derivative and ß-lactamase inhibitor with antibacterial activity. Tazobactam was discovered by Dr. R.G. Micetech at the University of Alberta in 1982. Tazobactam contains a ß-lactam ring and irreversibly binds to ß-lactamase at or near its active site. This protects other ß-lactam antibiotics from ß-lactamase catalysis. This compound is used in conjunction with ß-lactamase susceptible penicillins to treat infections caused by ß-lactamase producing organisms.
Tazobactam alone showed an MIC of ≤ 8 mg/liter (range 2 - 32 mg/liter) against several Acinetobacter baumannii strains. Tazobactam in combination with piperacillin, successfully restored the activity of piperacillin against β-lactamase-producing bacteria. Tazobactam exhibited inhibitory activity and protected piperacillin against Richmond and Sykes types II, III, IV and V β-lactamases, staphylococcal penicillinase and extended-spectrum β-lactamases. Tazobactam showed species-specific activity against class I chromosomally-mediated enzymes.
Tazobactam Sodium is soluble in water and methanol.
| Mechanism of Action | Tazobactam Sodium contains a ß-lactam ring that binds strongly to ß-lactamase at or near its activation site, thereby permanently inhibiting enzymatic activity. This action protects other ß-lactam antibiotics (penicillins, cephalosporins, etc.) from ß-lactamase catalysis, thereby enhancing their antibacterial activity. |
| Spectrum |
Tazobactam exhibits little useful antimicrobial activity, although weak activity against Acinetobacter spp. and Borrelia burgdorferi has been reported. Tazobactam inhibits a wide range of β-lactamases, including the group 2 penicillinases from Staphylococcus aureus, the TEM-1 and SHV-1 β-lactamases, many extended-spectrum enzymes, and the common group 2e cephalosporinases of Bacteriodes. fragilis. Against the group 1 cephalosporinases, activity is strongly influenced by the amount of enzyme produced. The inhibitor-resistant group 2br β-lactamases are poorly inhibited and group 3 metallo-β-lactamases are not inhibited at clinically useful levels. It is a poor inducer of β-lactamases of Gram-positive and Gram-negative organisms. |
| Impurity Profile | Related Substances: Single Impurity: Not more than 2.0% Total Impurities: Not more than 4.0% |
| Microbiology Applications | Tazobactam is often combined with the extended-spectrum β-lactam antibiotic piperacillin in the compound Zosyn or Tazocin (piperacillin/tazobactam), used in infections due to Pseudomonas aeruginosa. Tazobactam broadens the spectrum of piperacillin by making it effective against organisms that express β-lactamase and would normally degrade piperacillin. |
| Molecular Formula | C10H11N4NaO5S |
| References |
Hertz FB et al (2022) Efficacy of piperacillin-tazobactam and cefotaxime against Escherichia coli hyperproducing TEM-1 in a mouse peritonitis infection model. Int. J. Antimicrob. Agents 59(4) 106543 Wishart D (2005) Tazobactam. DrugBank. The Metabolomics Innovation Center. |