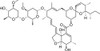
Delta-2-Avermectin B1a is a natural, irreversible base degradation product produced by selective hydrolysis of the terminal saccharide Avermectin under acidic conditions in animals and in the environment. Delta-2-Avermectin B1a is formed by rearrangement of the naturally-occurring delta-3-group to the 2-position. Although less active than the parent, Delta-2-Avermectin B1a is nonetheless active at 1 ppm. Avermectins are a family of natural macrocyclic lactones produced by the soil actinomycete Streptomyces avermitilis. There are eight different Avermectin natural product compounds: A1a, A1b, A2a, A2b, B1a, B1b, B2a, and B2b based upon the structure of the individual compounds. The B1 fraction has the most effective antiparasitic activity.
Delta-2-Avermectin B1a is soluble in ethanol, methanol, DMF and DMSO.
| Mechanism of Action | Avermectins can modulate gamma-aminobutyric acid (GABA) chloride channels in vertebrate neurons. |
| Insect Biology Applications | Electrophysiological findings by injection of E. elegans mRNA into Xenopus laevis oocytes indicated that Avermectins act on glutamate-gated chloride channels in nematodes. |
| References |
Blizzard T, Fisher MH, Mrozik H, Shih TL (1990) Avermectins and Milbemycins. In: Lukacs G, Ohno M. (eds) Recent progress in the chemical synthesis of antibiotics. Springer, Berlin, Heidelberg Chabala JC et al (1980) Ivermectin, a new broad-spectrum antiparasitic agent. J. Med. Chem. 23:1134 Chen TS and Inamine ES (1989) Studies on the biosynthesis of Avermectins. Arch Biochem Biophys. 270(2):521-5 PMID 2705778 Egerton JR et al (year) Avermectins, new family of potent anthelmintic agents: Efficacy of the B1a component. Antimicrob. Agents Chemother. 15(3):372-378 PMID 464563 Ikeda H and Omura S (1997) Avermectin biosynthesis. Chem. Rev. 97(7):2591-2610 Mrozik H et al (1982) Avermectin aglycones. 47:489 Pivnichny JV, Arison BY, Preiser FA, Shim JSK and Mrozik H (1988) Base-catalyzed isomerization of avermectins. J. Agric. Food Chem 36(4):826-828 Pivnichny JV, Shim JK and Zimmerman LA (1983) Direct determination of avermectins in plasma at nanogram levels by high-performance liquid chromatography. J. Pharm. Sci. 72(12):1447-1450 PMID 464563 |