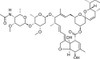
Eprinomectin B1a is a semi-synthetic analogue of avermectin B1a prepared by oxidation of the 4''-hydroxy moiety and reductive amination followed by acetylation. Eprinomectin B1a is the major component (>90%) of the commercial product for endo- and exo-parasite control, eprinomectin. The avermectins and milbemycins are also potent insecticides and acaricides.
Eprinomectin B1a is soluble in ethanol, methanol, DMF or DMSO. Poor water solubility.
| Mechanism of Action | Members of the avermectin/milbemycin class exert their anthelmintic effects by binding to glutamate-gated chloride channels expressed on nematode neurons and pharyngeal muscle cells. |
| References |
Cvetovich RJ et al (1994) Synthesis of 4"-epi-amino-4"-deoxyavermectins B1. J. Org. Chem. 59:7704 Mrozik HH et et al. 4-Keto- and 4-amino-4-deoxy avermectin compounds and substituted amino derivatives thereof. 984 US Patent 4,427,663 |