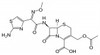
Cefotaxime Sodium, USP is broad-spectrum, third-generation cephalosporin. It interferes with bacterial peptidoglycan synthesis. Cefotaxime Sodium is freely soluble in aqueous solution.
Cefotaxime Sodium, USP conforms to United States Pharmacopoeia specifications.
We also offer:
- Cefotaxime ReadyMadeTM Solution (C293)